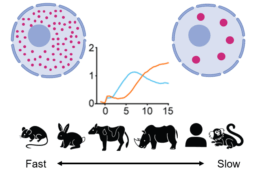
Role of surface condensation for the assembly of cortical proteins (A1)
Objective
The goal of A1 is to understand how surface condensation of scaffold proteins at biological membranes can control the patterning and the mechanics of an acto-myosin cell cortex.
Research Description
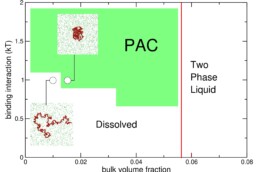
The formation of epithelial tissue requires precise spatiotemporal control of cell surface properties such as formation of adhesion complexes that are linked to the cell cortex at the level of the cell membrane1,2. The Honigmann group has discovered that surface condensation of ZO scaffold proteins at the membrane is a mechanism that cells exploit to pattern adhesion complexes and the actin cortex3. In vitro reconstitutions have shown that ZO surface condensates can nucleate and bundle actin fibers, which can drive the formation of a variety of cortical patterns4-6. How ZO surface condensation and actin polymerization are linked and what mechanical properties emerge from this is not understood.
Research questions
How does surface condensation of ZO proteins induce actin recruitment and polymerization? and What are the mechanical properties of a condensed ZO1-actin cortex on the membrane?
Thesis Project Topic
Linking surface condensation of scaffold proteins to the assembly of cell junctions
Training
The PhD students will be trained in protein and membrane biochemistry methods including membrane reconstitutions, biophysical methods such as fluorescence fluctuation spectroscopy, in addition to super-resolution microscopy and image analysis methods.
Profile of Prospective Students
- Candidates have a Masters degree in biophysics or related fields
- Candidates are expected to have a solid basis in physics, biology, or related fields.
- Experience in fluorescence microscopy is plus

Supervisor: Alf Honigmann
Membrane Organization of Cells and Tissues
Discipline: Biophysics
Affiliation: Biotec (TU-Dresden) | Physics of Life (TU Dresden)
Contact: alf.honigmann (at) tu-dresden (dot) de
Lab Webpage
References
- Roignot J, Peng X, Mostov K. Polarity in Mammalian Epithelial Morphogenesis. Cold Spring Harb Perspect Biol. 2013;5(2):a013789-a013789. https://doi.org/10.1101/cshperspect.a013789
- Mukenhirn M, Wang CH, Guyomar T, Bovyn MJ, Staddon MF, van der Veen RE, Maraspini R, Lu L, Martin-Lemaitre C, Sano M, Lehmann M, Hiraiwa T, Riveline D, Honigmann A. Tight junctions control lumen morphology via hydrostatic pressure and junctional tension. Dev Cell. https://doi.org/10.1016/j.devcel.2024.07.016
- Beutel O, Maraspini R, Pombo-García K, Martin-Lemaitre C, Honigmann A. Phase Separation of Zonula Occludens Proteins Drives Formation of Tight Junctions. Cell. 2019;179(4):923-936.e11. https://doi.org/10.1016/j.cell.2019.10.011
- Zhao X, Bartolucci G, Honigmann A, Jülicher F, Weber CA. Thermodynamics of wetting, prewetting and surface phase transitions with surface binding. New J Phys. 2021;23(12):123003. https://doi.org/10.1088/1367-2630/ac320b
- Pombo-García, K, Adame-Arana, O, Martin-Lemaitre, C, Jülicher, F, Honigmann, A. Membrane prewetting by condensates promotes tight junction belt formation. Nature. 2024; 632, 647–655. https://doi.org/10.1038/s41586-024-07726-0
- Sun, D, Zhao, X, Wiegand, T, Bartolucci, G, Martin-Lemaitre, C, Grill, S, Hyman, AA, Weber, C, Honigmann, A. Assembly of tight junction belts by surface condensation and actin elongation. bioRxiv. Published online 2023. https://doi.org/10.1101/2023.06.24.546380
Explore other RTG Thesis Projects
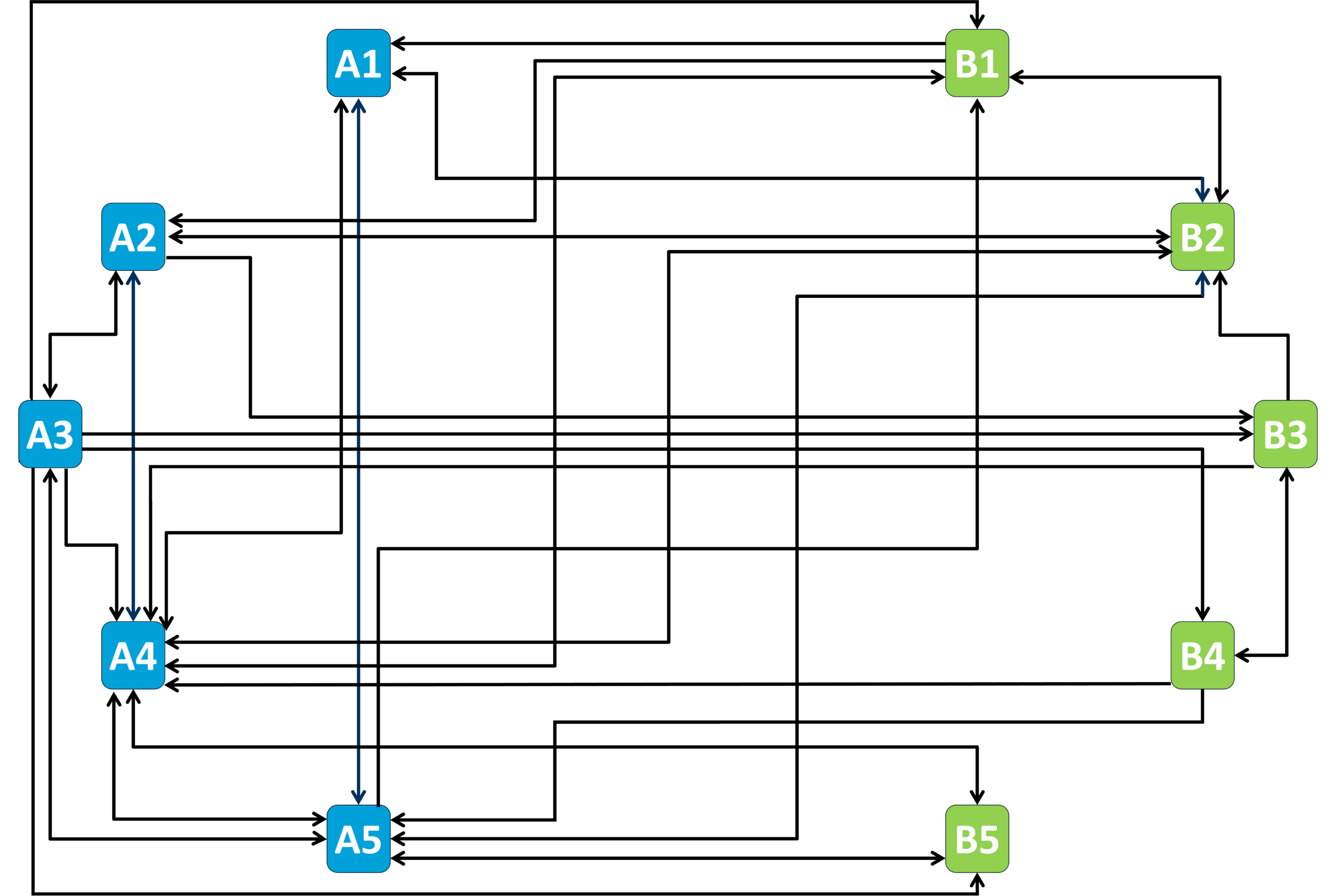
Collaborations within the RTG
Click on the different project numbers (e.g. A1) to find out more about the theme of their ongoing collaborations and explore the project details
A2 - Biomolecular condensate regulation (Harmon)
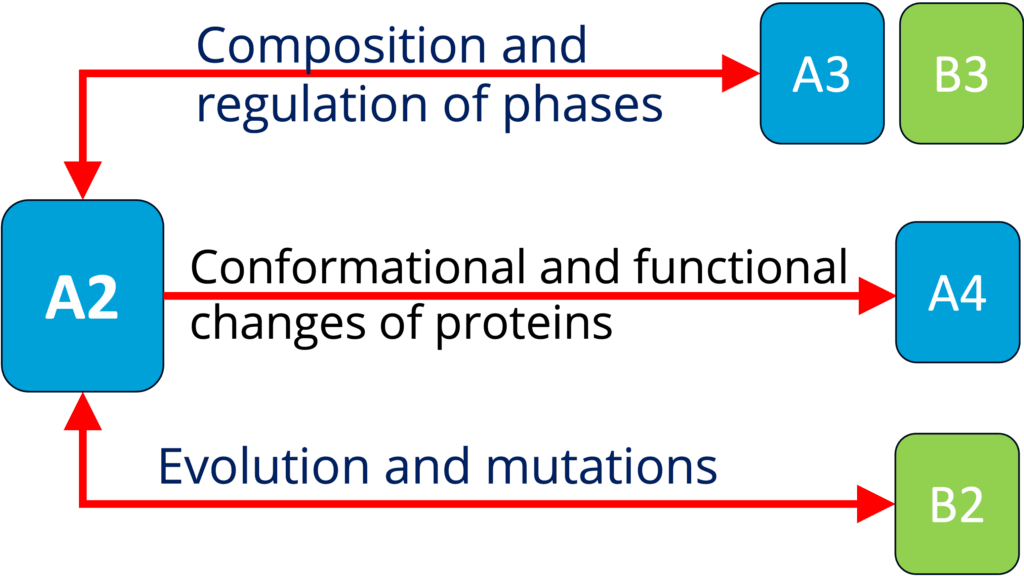
See project details: https://dresdencondensates.org/projects/a2/
A4 - Theory and simulation of polymer-assisted condensates (Sommer)
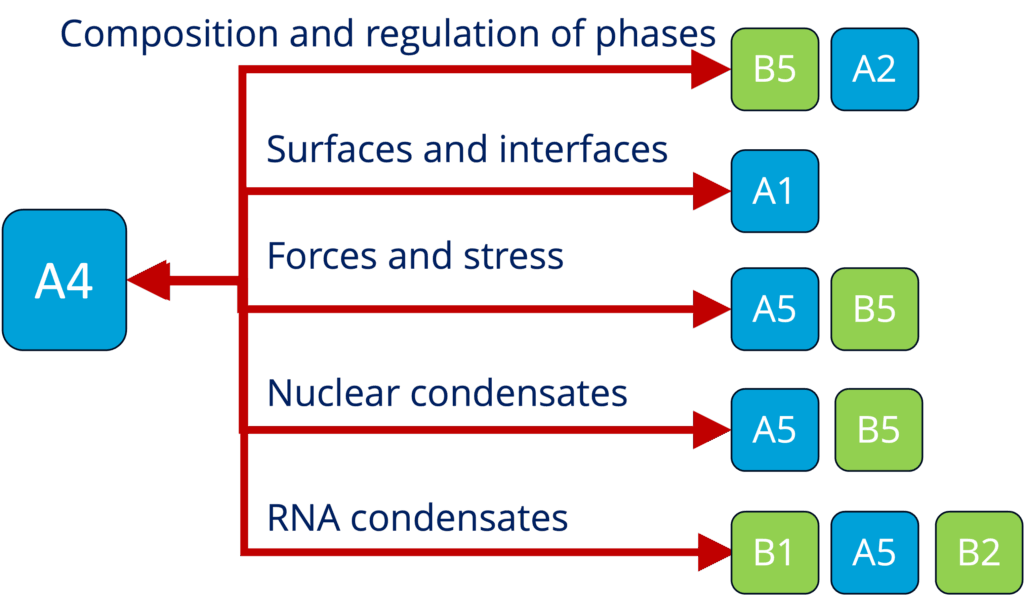
See project details: https://dresdencondensates.org/projects/a4/
B2 - Characterizing the role of RNP granules in ALS (Sterneckert)
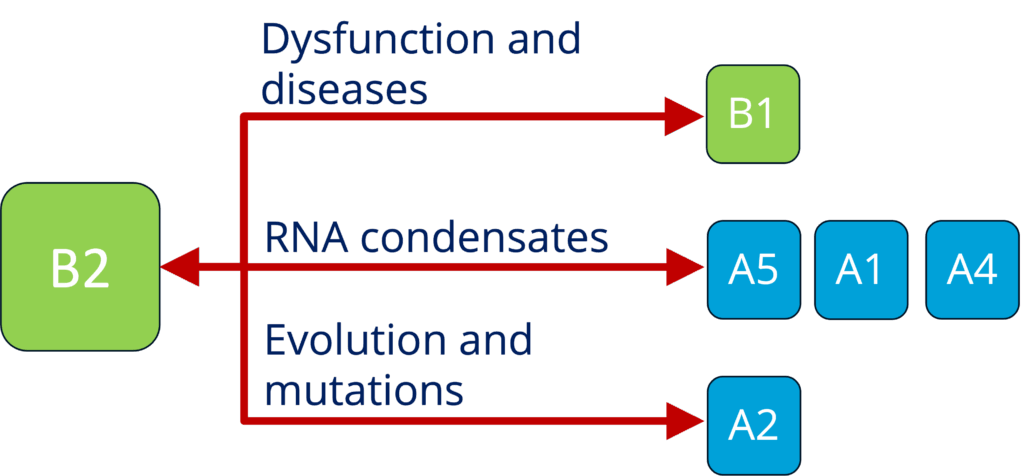
See project details: https://dresdencondensates.org/projects/b2/
A1 - Role of surface condensation for the assembly of cortical proteins (Honigmann)
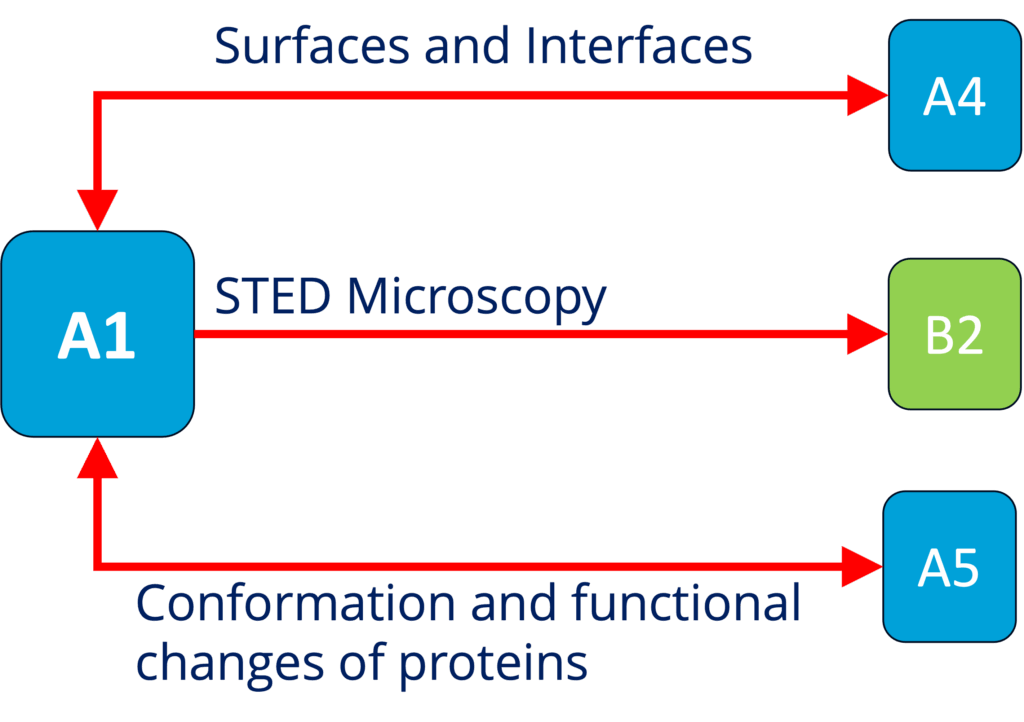
See project details: https://dresdencondensates.org/projects/a1/
A3 - Spectroscopy and local interactions in condensates and organization of the cytoplasm (Adams)
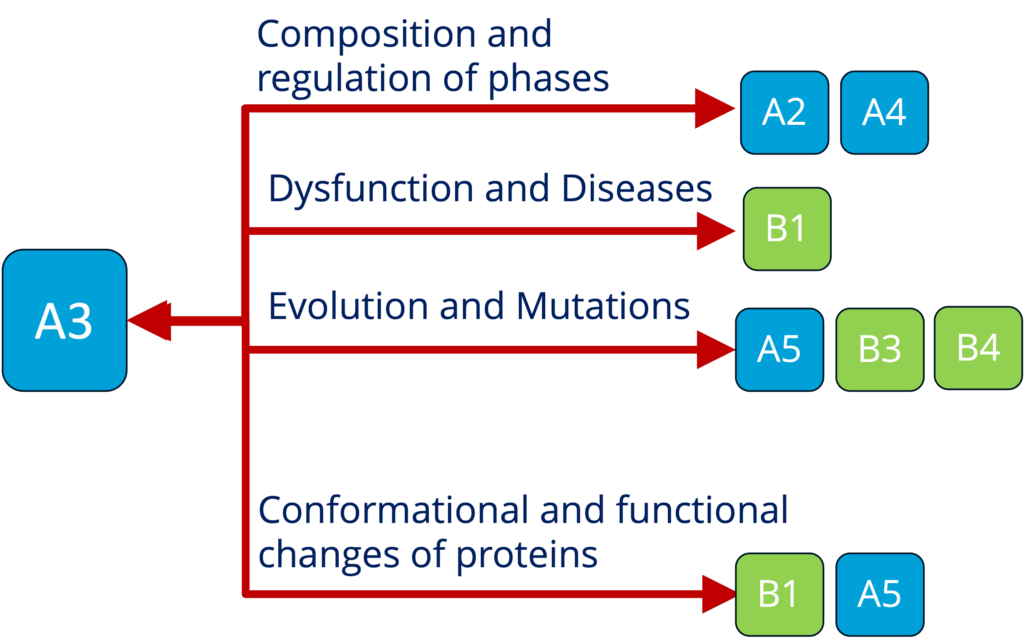
See project details: https://dresdencondensates.org/projects/a3/
A5 - Capillary forces and the force response of condensates (Jahnel and Grill)
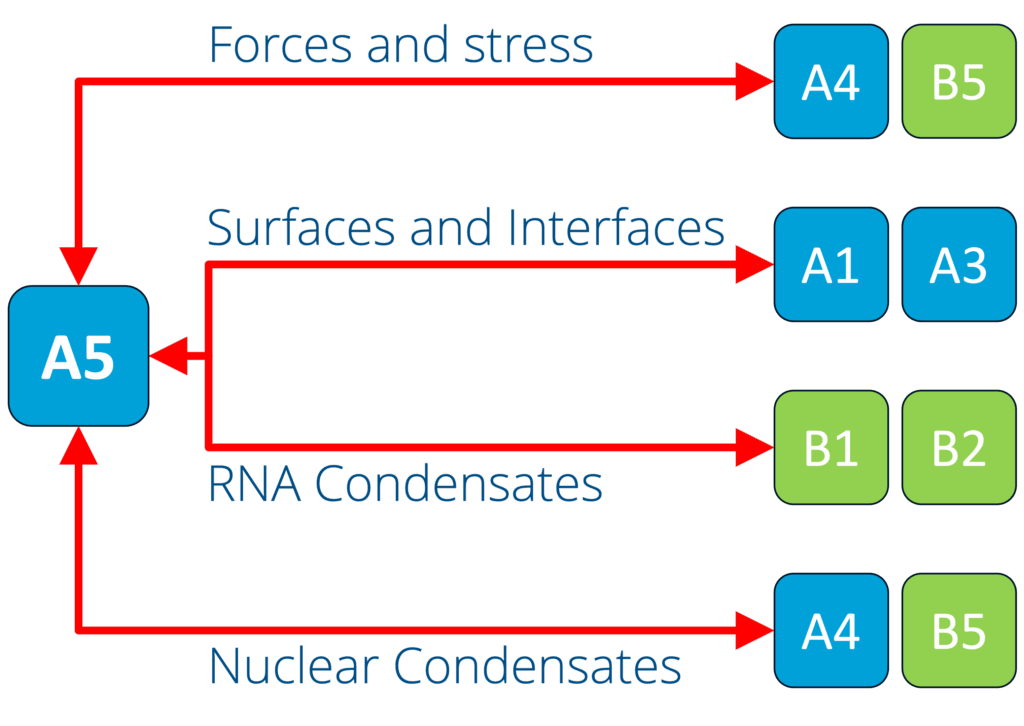
See project details: https://dresdencondensates.org/projects/a5/
B1 - Elucidating the mechanisms underlying mRNA translation regulation by condensation (biophysics and biochemistry) (Alberti and Schlierf)
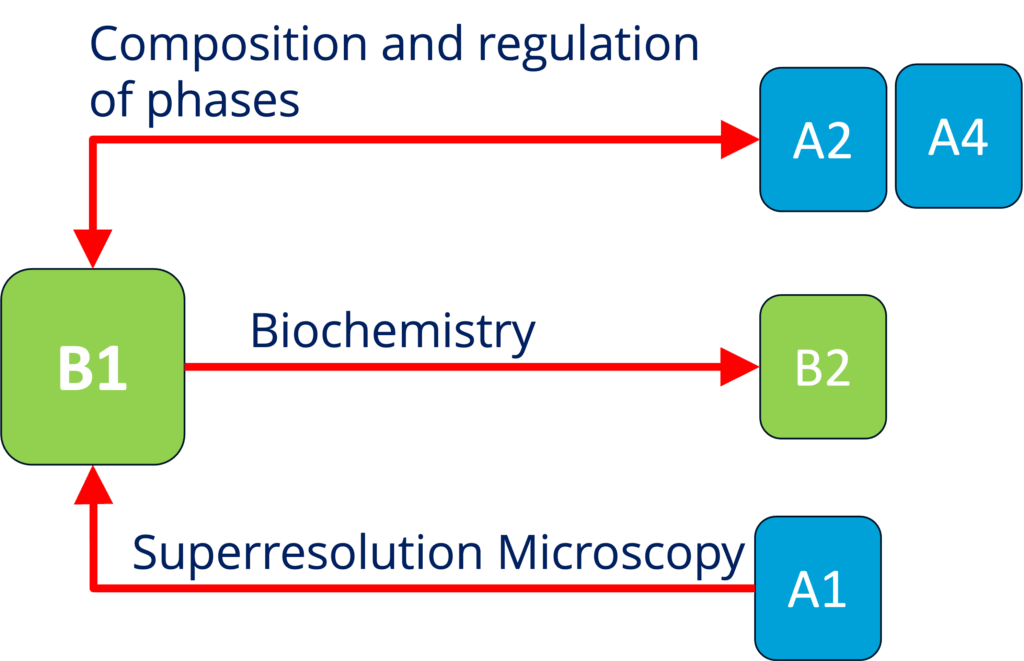
See project details: https://dresdencondensates.org/projects/b1/
B3 - Sequence to function mapping of condensate proteomes (Toth-Petroczy)
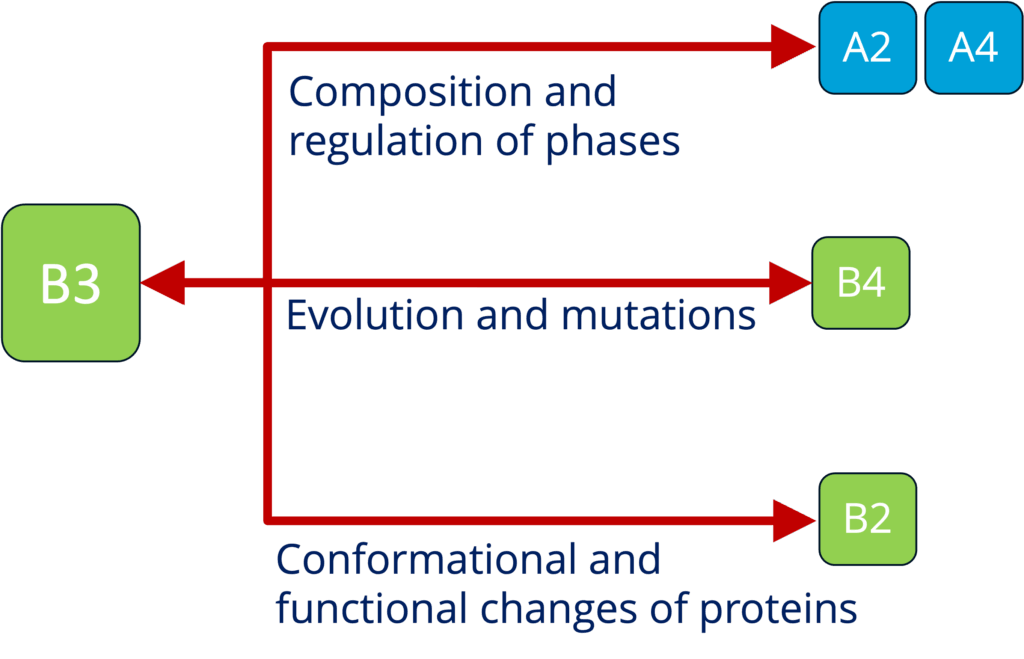
See project details: https://dresdencondensates.org/projects/b3/
B4 - Role of condensates in biological time across mammals (Ebisuya and Hyman)
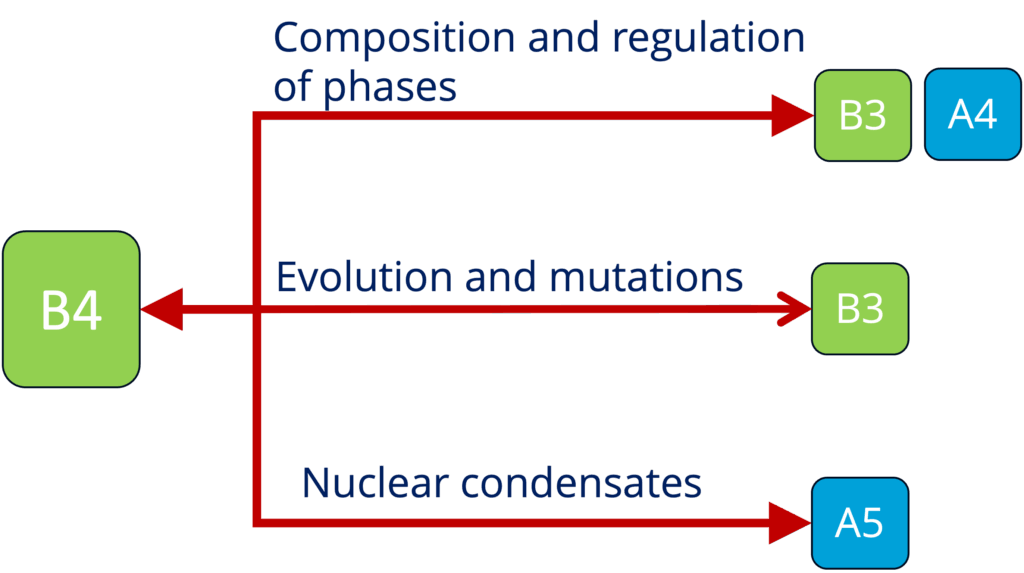
See project details: https://dresdencondensates.org/projects/b4/
B5 - Role of condensates in epigenetics (experiments and theory) (Brugués and Schiessel)
See Project Details: https://dresdencondensates.org/projects/b5/