Characterizing the role of RNP granules in ALS (B2)
Objective
The goal of B2 is to understand the role of RNP granules in the pathogenesis of amyotrophic lateral sclerosis (ALS).
Project Description
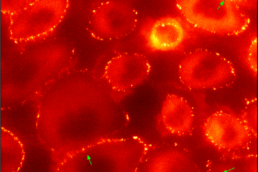
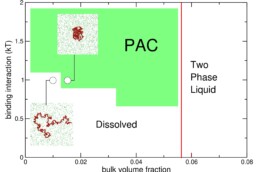
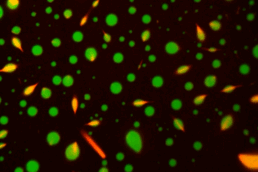
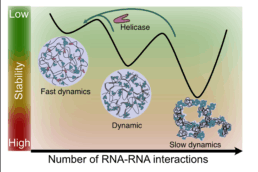
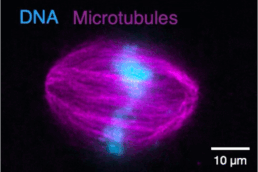
Motor neurons (MNs) have very long axons that require local translation of mRNAs transported from the soma via ribonucleoprotein (RNP) granules. RNP granules are condensates that protect mRNA from degradation in a translationally arrested state1. There are many different types of RNP granules found in MN axons, and MNs are able to disassemble individual granules at specific times and locations in order to support specialized functions such as metabolism or injury repair1. Highlighting the importance of this process, alterations in RNP granules have been linked to ALS pathogenesis and MN degeneration2,3. Thus, a better understanding of RNP granules could lead to novel therapeutics to protect MN axons against degeneration in ALS. To reach this objective, the Sterneckert team uses induced pluripotent stem cell (iPSC)-derived MNs4-6 to investigate RNP granule dynamics in a physiological model. In collaboration with the Hyman and Alberti teams, we generated FUS-eGFP reporter iPSCs for live-cell imaging of RNP granules, facilitating compound screening to identify novel drugs for repurposing as ALS therapeutics4. We also generated a microfluidic device enabling the study of axonal RNP granules as well as the functionality of neuromuscular junctions6. Using this technology, we uncovered evidence that mutant FUS disrupts axonal RNP granules, leading to reduced translation of nuclear proteins required for axonal mitochondrial function (see figure). Here, we will use these technologies to answer fundamental questions about RNP granules in MNs and how this is impacted by ALS pathogenesis.

Research questions
Project 1:
- How are multiple different RNP granules with individualized mRNA cargo maintained in MN axons?
- How much exchange of RNA-binding proteins occurs between different RNP granules within axons?
- How do RNP granules regulate their size and prevent fusion in order to maintain separation of mRNAs?
Project 2:
- How does ALS pathogenesis impact RNP granule maintenance and composition?
To answer these research questions, both projects will utilize dCas13d-mediated proximity labeling to identify the proteins binding specific axonal mRNAs. Pulse-chase experiments can be used to assess if proteins in RNP granules are exchanged over time. Project 2 will compare motor neurons derived from isogenic iPSCs to characterize the impact of ALS mutations. In addition, we will explore if specific oligonucleotides (“bait RNAs”) could offer an effective strategy for reversing ALS pathogenesis.
Thesis Project Topic
Understanding how human motor neuron axons maintain diverse RNP granule subtypes
Training
The PhD students will be trained in iPSC culture, MN differentiation, confocal microscopy, live cell imaging, and image analysis. Collaborations with groups using alternative model systems, including theoretical groups, will further the acquisition of communication skills and interdisciplinary thinking.
Profile of Prospective Students
- Candidates have a Masters degree in biology or related fields
- Candidates should have a sound basis in cell biology, neurology or closely related fields.
- Experience in microscopy and cell culture are expected

Supervisor: Jared Sterneckert
iPS Cells and Neurodegenerative Diseases
Discipline: Biology / Neurodegenerative Diseases
Affiliation: Center for Regenerative Therapies Dresden (CRTD – TU Dresden)
Contact: jared.sterneckert (at) tu-dresden (dot) de
References
- Dalla Costa, I., Buchanan, C. N., Zdradzinski, M. D., Sahoo, P. K., Smith, T. P., Thames, E., Kar, A. N. & Twiss, J. L. The functional organization of axonal mRNA transport and translation. Nat Rev Neurosci 22, 77-91 (2021). DOI: 10.1038/s41583-020-00407-7
- Li, Y. R., King, O. D., Shorter, J. & Gitler, A. D. Stress granules as crucibles of ALS pathogenesis. Journal of Cell Biology 201, 361-372 (2013). DOI: 10.1083/jcb.201302044
- Altman, T., Ionescu, A., Ibraheem, A., Priesmann, D., Gradus-Pery, T., Farberov, L., Alexandra, G., Shelestovich, N., Dafinca, R., Shomron, N., Rage, F., Talbot, K., Ward, M. E., Dori, A., Kruger, M. & Perlson, E. Axonal TDP-43 condensates drive neuromuscular junction disruption through inhibition of local synthesis of nuclear encoded mitochondrial proteins. Nat Commun 12, 6914 (2021). DOI: 10.1038/s41467-021-27221-8
- Marrone, L., Poser, I., Casci, I., Japtok, J., Reinhardt, P., Janosch, A., Andree, C., Lee, H. O., Moebius, C., Koerner, E., Reinhardt, L., Cicardi, M. E., Hackmann, K., Klink, B., Poletti, A., Alberti, S., Bickle, M., Hermann, A., Pandey, U. B., Hyman, A. A. & Sterneckert, J. L. Isogenic FUS-eGFP iPSC Reporter Lines Enable Quantification of FUS Stress Granule Pathology that Is Rescued by Drugs Inducing Autophagy. Stem Cell Reports 10, 375-389 (2018). DOI: 10.1016/j.stemcr.2017.12.018
- Marrone, L., Drexler, H. C. A., Wang, J., Tripathi, P., Distler, T., Heisterkamp, P., Anderson, E. N., Kour, S., Moraiti, A., Maharana, S., Bhatnagar, R., Belgard, T. G., Tripathy, V., Kalmbach, N., Hosseinzadeh, Z., Crippa, V., Abo-Rady, M., Wegner, F., Poletti, A., Troost, D., Aronica, E., Busskamp, V., Weis, J., Pandey, U. B., Hyman, A. A., Alberti, S., Goswami, A. & Sterneckert, J. FUS pathology in ALS is linked to alterations in multiple ALS-associated proteins and rescued by drugs stimulating autophagy. Acta Neuropathol 138, 67-84 (2019). DOI: 10.1007/s00401-019-01998-x
- Bellmann, Sterneckert et al. A customizable microfluidic platform for medium-throughput modeling of neuromuscular circuits. Biomaterials 225, 119537 (2019). DOI: 10.1016/j.biomaterials.2019.119537
Explore other RTG Thesis Projects
Collaborations within the RTG
Click on the different project numbers (e.g. A1) to find out more about the theme of their ongoing collaborations and explore the project details
A2 - Biomolecular condensate regulation (Harmon)
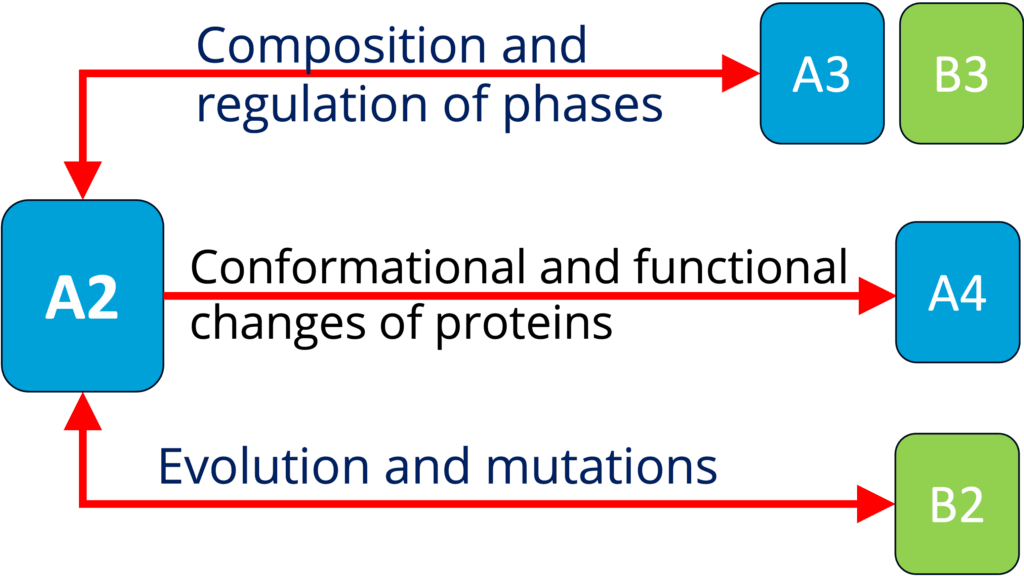
See project details: https://dresdencondensates.org/projects/a2/
A4 - Theory and simulation of polymer-assisted condensates (Sommer)
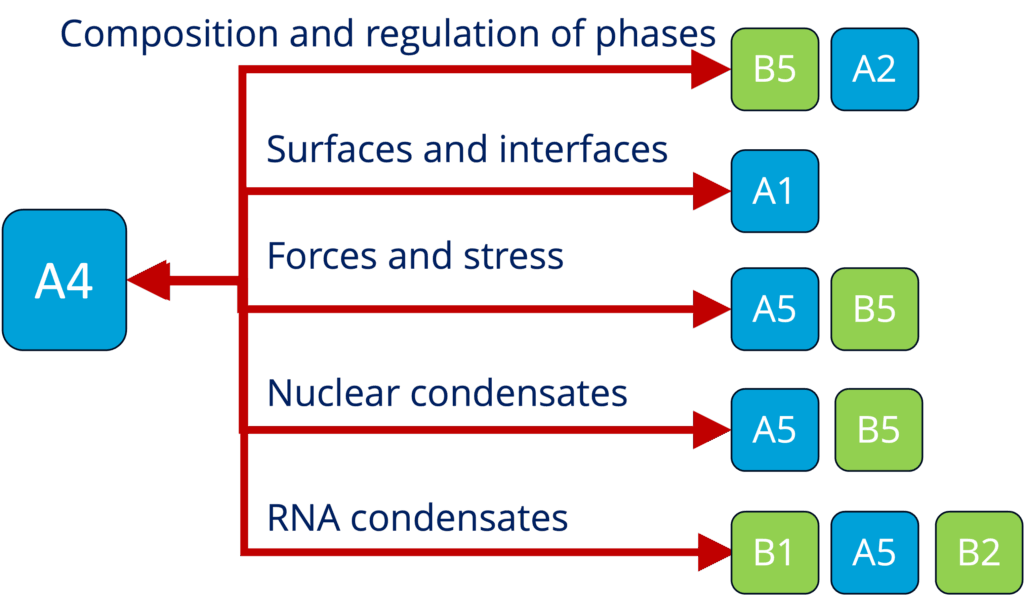
See project details: https://dresdencondensates.org/projects/a4/
B2 - Characterizing the role of RNP granules in ALS (Sterneckert)
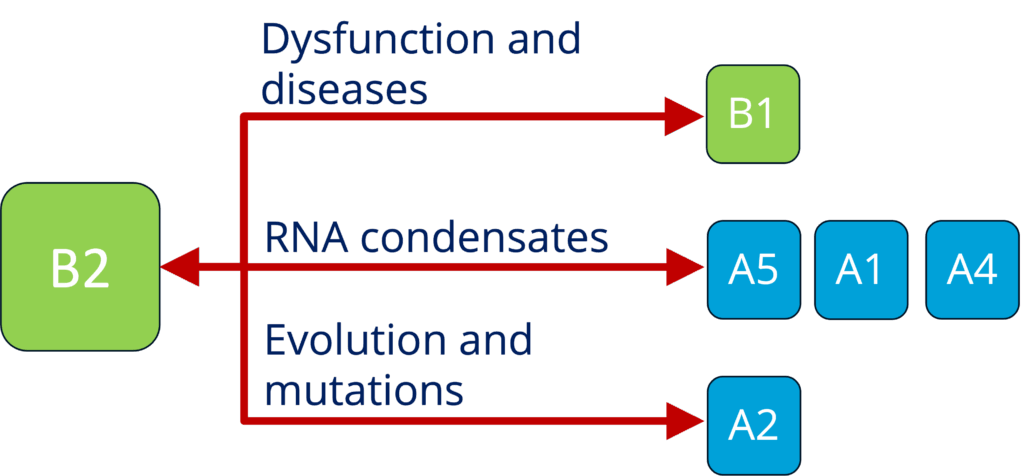
See project details: https://dresdencondensates.org/projects/b2/
A1 - Role of surface condensation for the assembly of cortical proteins (Honigmann)
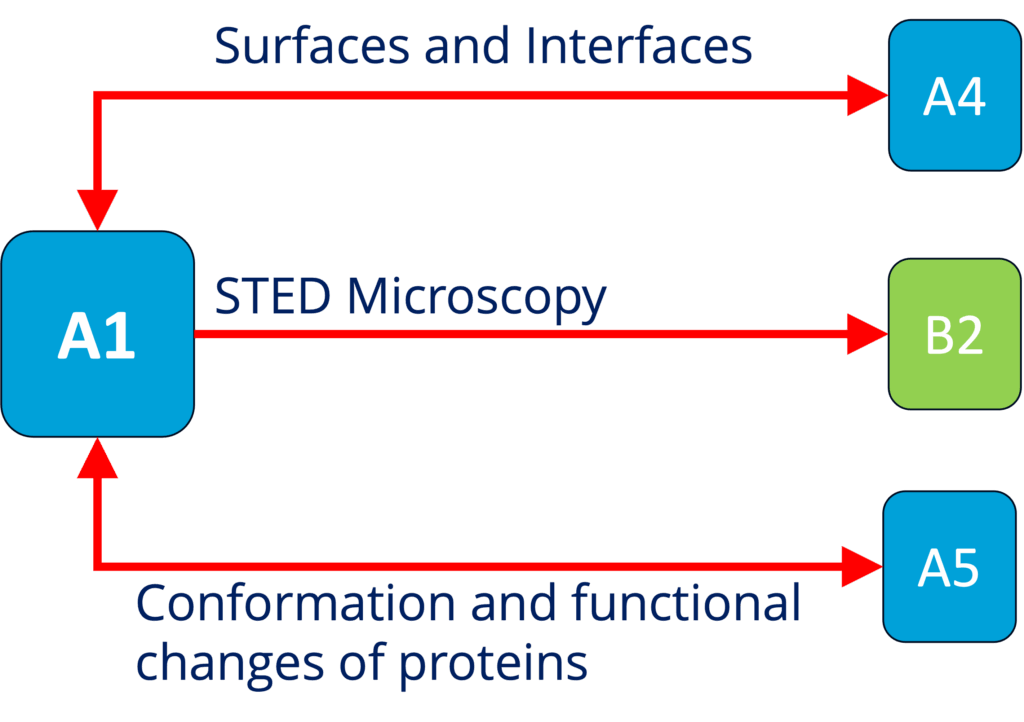
See project details: https://dresdencondensates.org/projects/a1/
A3 - Spectroscopy and local interactions in condensates and organization of the cytoplasm (Adams)
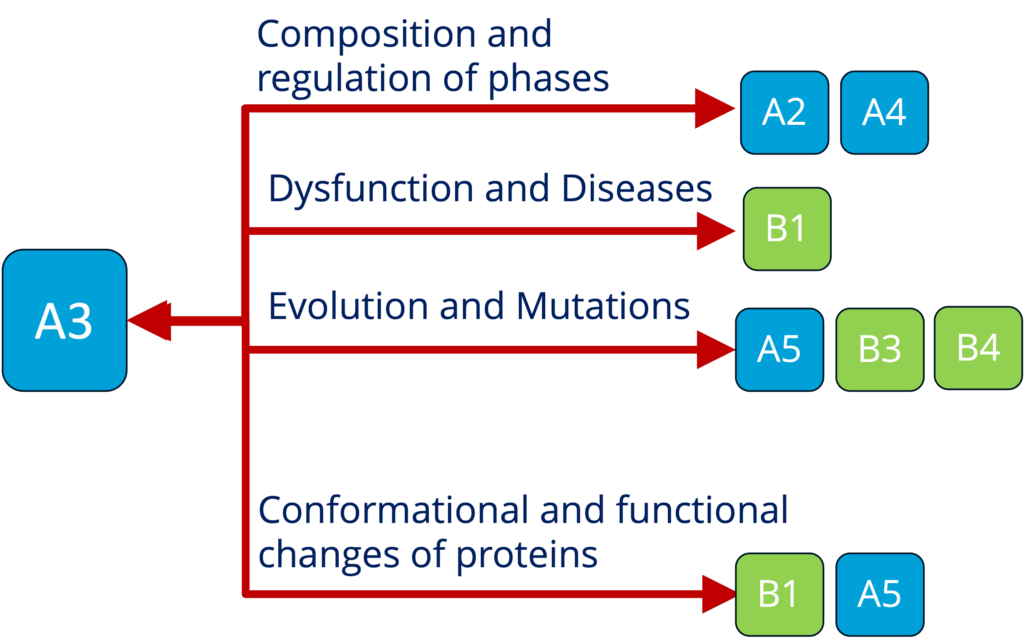
See project details: https://dresdencondensates.org/projects/a3/
A5 - Capillary forces and the force response of condensates (Jahnel and Grill)
See project details: https://dresdencondensates.org/projects/a5/
B1 - Elucidating the mechanisms underlying mRNA translation regulation by condensation (biophysics and biochemistry) (Alberti and Schlierf)
See project details: https://dresdencondensates.org/projects/b1/
B3 - Sequence to function mapping of condensate proteomes (Toth-Petroczy)
See project details: https://dresdencondensates.org/projects/b3/
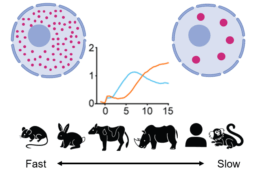
B4 - Role of condensates in biological time across mammals (Ebisuya and Hyman)
See project details: https://dresdencondensates.org/projects/b4/
B5 - Role of condensates in epigenetics (experiments and theory) (Brugués and Schiessel)
See Project Details: https://dresdencondensates.org/projects/b5/