A role for RNA in Stress Granules assembly
Stress granules are membraneless compartments formed by phase separation of specific molecules upon exposure to cellular stress such as oxidative stress, heat shock, or osmotic stress.
The Alberti, Jahnel, Honigmann, and Hyman labs published a study in cell highlighting the role of RNA in the assembly of stress granules by crosslinkinig with G3BP clusters and how G3BP clusters in return prevent RNA entanglement. The study entitled “RNA-Induced Conformational Switching and Clustering of G3BP Drive Stress Granule Assembly by Condensation” is in collaboration with Washington University, the European Molecular Biology Laboratory, Heidelberg, and Pohang University of Science and Technology, Korea.
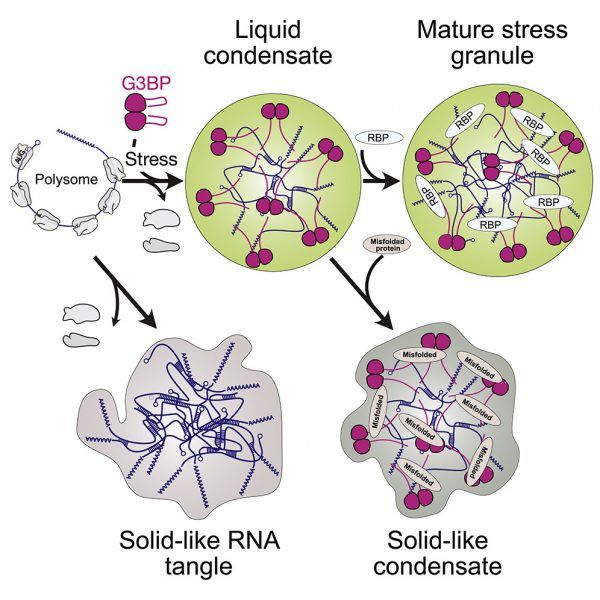
Graphical Abstract:
Stressed cells shut down translation, release mRNA molecules from polysomes, and form stress granules (SGs) via a network of interactions that involve G3BP. Here we focus on the mechanistic underpinnings of SG assembly. We show that, under non-stress conditions, G3BP adopts a compact auto-inhibited state stabilized by electrostatic intramolecular interactions between the intrinsically disordered acidic tracts and the positively charged arginine-rich region. Upon release from polysomes, unfolded mRNAs outcompete G3BP auto-inhibitory interactions, engendering a conformational transition that facilitates clustering of G3BP through protein-RNA interactions. Subsequent physical crosslinking of G3BP clusters drives RNA molecules into networked RNA/protein condensates. We show that G3BP condensates impede RNA entanglement and recruit additional client proteins that promote SG maturation or induce a liquid-to-solid transition that may underlie disease. We propose that condensation coupled to conformational rearrangements and heterotypic multivalent interactions may be a general principle underlying RNP granule assembly.
Read the full publication
https://www.sciencedirect.com/science/article/pii/S0092867420303421